

However, the first arrangement of atoms is preferred because it has the lowest number of atoms with nonzero formal charges (Guideline 2). Note that the sum of the formal charges in each case is equal to the charge of the ion (–1). Possible Lewis structures and the formal charges for each of the three possible structures for the thiocyanate ion are shown here: The formal charges present in each of these molecular structures can help us pick the most likely arrangement of atoms. We can draw three possibilities for the structure: carbon in the center and double bonds, carbon in the center with a single and triple bond, and oxygen in the center with double bonds:Ĭomparing the three formal charges, we can definitively identify the structure on the left as preferable because it has only formal charges of zero (Guideline 1).Īs another example, the thiocyanate ion, an ion formed from a carbon atom, a nitrogen atom, and a sulfur atom, could have three different molecular structures: CNS –, NCS –, or CSN –. We know from our previous discussion that the less electronegative atom typically occupies the central position, but formal charges allow us to understand why this occurs. To see how these guidelines apply, let us consider some possible structures for carbon dioxide, CO 2.
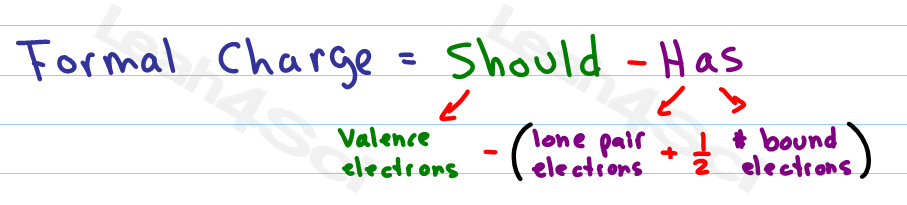
With the oxidation state formalism, the electrons in the bonds are "awarded" to the atom with the greater electronegativity. This can be most effectively visualized in an electrostatic potential map. The covalent (sharing) aspect of the bonding is overemphasized in the use of formal charges, since in reality there is a higher electron density around the oxygen atoms due to their higher electronegativity compared to the carbon atom. The formal charge view of the CO 2 molecule is essentially shown below: With formal charge, the electrons in each covalent bond are assumed to be split exactly evenly between the two atoms in the bond (hence the dividing by two in the method described above). The reason for the difference between these values is that formal charges and oxidation states represent fundamentally different ways of looking at the distribution of electrons amongst the atoms in the molecule.

If the formal charges and oxidation states of the atoms in carbon dioxide are compared, the following values are arrived at: The concept of oxidation states constitutes a competing method to assess the distribution of electrons in molecules. \)įormal charge compared to oxidation stateįormal charge is a tool for estimating the distribution of electric charge within a molecule.


 0 kommentar(er)
0 kommentar(er)
